05.11.2020
New fuels for carbon free power production
The BIGH2 project develops advanced combustion technology that utilizes mixtures of hydrogen and ammonia as carbon-free fuel in industrial gas turbines.
Engines that can use carbonfree fuels before 2030
GAS TURBINE MANUFACTURERS aim to deliver engines that can use carbonfree fuels before 2030. The gas turbines must have the same level of performance as today’s state-of-the-art equipment, powered by natural gas, and meet current requirements regarding emissions of nitrogen oxides and other pollutants.
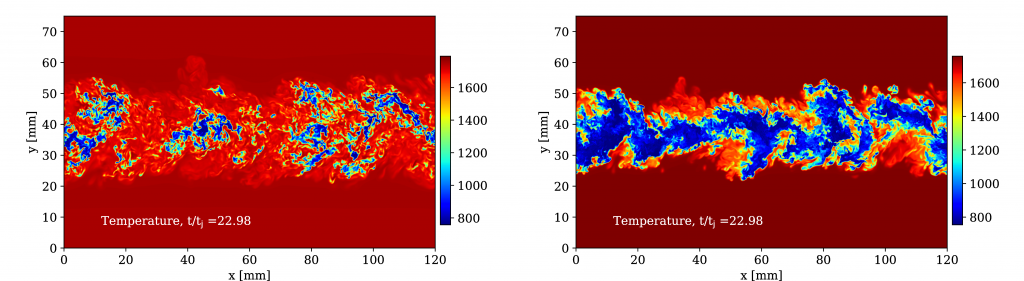
The project explores key challenges related to the use hydrogen and ammonia as gas turbine fuels: to ensure a stable flame in the combustion chamber and avoid emissions of
nitrogen oxides that exceed regulatory standards. Hydrogen represents, in many respects, an optimal fuel because it burns easily in a very stable fashion and, if certain combustion conditions are satisfied, also very cleanly as its only combustion product is water vapour.
However, hydrogen also poses important logistical challenges because the gas is highly explosive and characterized by very low energy density. Therefore, it’s problematic to store hydrogen safely in very large quantities.
A convenient hydrogen and energy carrier
The BIGH2 project investigates the possibility of using ammonia (NH3) as a hydrogen carrier because ammonia is considerably easier to handle than hydrogen.
While hydrogen is a relatively new fuel in the transportation and power generation sectors, still lacking well-established logistics and infrastructure, ammonia is already
produced, transported and stored in large quantities for the manufacture of fertilizers, for example.
“Ammonia is a very well-known chemical, and we draw on comprehensive, accumulated experience from all links of the value chain. An extensive distribution network is already in place, and we have effective systems and safety mechanisms for handling the chemical,” says project director Andrea Gruber at SINTEF.
So, how could ammonia be used as an energy carrier in the production of power with large gas turbines?
Upgrading ammonia
The challenge with ammonia is that it’s a rather poor fuel, especially for gas turbines, characterized by weak ignition and combustion properties and by a rather obnoxious tendency to form atmospheric pollutants (nitrogen oxides, NOx) in the exhaust. Hence,
BIGH2 aims to find out if blending ammonia and hydrogen can result in a better gas turbine fuel than neat (pure) ammonia. A fuel mixture consisting of hydrogen, nitrogen and ammonia can be readily obtained by cracking the ammonia molecule into hydrogen and nitrogen by heating the chemical in a catalyst. Vast amounts of waste heat are often available in gas turbines as bi-product of the combustion process.
“Before sending the ammonia into the gas turbine, the chemical compound can be cracked, completely or partially, by using surplus heat – and using the resulting hydrogen, which is a highly reactive element, to upgrade the fuel mixture,” says Gruber.
“In this project, we try to understand how different blends of ammonia, hydrogen and nitrogen behave in the combustion chamber, compared to natural gas.”
It’s important to find the “sweet spot” and limit the cracking of ammonia to ensure a stable and clean combustion. This is also important because the cracking process itself uses surplus heat from the gas turbine, and this heat has alternative utility value for steam turbine cycles or district heating. Therefore, the intention of the project is to identify the optimal mixture of hydrogen, nitrogen and ammonia that requires as little cracking of ammonia as possible.
NOx reduction
Another important challenge presented by the combustion of ammoniacontaining fuels, is that toxic nitrogen oxides (NOx) are a by-product of the process.
“So our focus is, beside flame stability, also on minimizing the formation of NOx in the combustion chamber,” says Gruber. “We are trying to identify the operating conditions that produce the lowest level of NOx, for instance by organizing the chemical reaction process in different stages within the combustion chamber.
But, if this strategy fails, we are also considering using some of the ammonia as a reduction agent for NOx abatement in the exhaust gas.”
The researchers investigate the challenges from different angles.
“At SINTEF, we are trying to deepen our knowledge of the basic combustion process. We have modelled the flames of hydrogen and ammonia, in order to better understand the effect of these new fuel mixtures. This has required a comprehensive and coordinated effort together with our research partners, NTNU in Trondheim and Sandia National Laboratories in California,” says Gruber.
Laboratory experiments have also been conducted utilizing SINTEF’s pressurized combustion rig (HIPROX) at Gløshaugen, where a downscaled version of a gas turbine burner is used to evaluate flame stability and NOx reduction strategies.
Close cooperation
“We are using a very complex and advanced Siemens gas turbine burner, where the combustion process is compartmentalized into three stages.
We are conducting tests with different compositions of the fuel in the different
stages. For instance, we can inject undiluted ammonia in one stage and add cracked ammonia in the other two, trying to find the optimal operation point.”
At the same time, NTNU conducts experiments on simpler flames using less complicated burners compared to the Siemens’ design and these experiments have spawned valuable fundamental knowledge on how these new fuel blends behave compared to natural gas.
“Ours is a fine group of partners. The combination of different research groups at SINTEF and NTNU, and the industrial locomotives Siemens and Equinor – both having concrete plans to develop hydrogen and ammonia as energy carriers – has been of immense value to the project. Sandia National Laboratories, the US Department of Energy leading research lab in the field of energy technologies, is involved in the project as an external associated partner. Together we are maturing a technology that now needs a small push to become a reality,” says Gruber
